Cancer
Study confirms link between breast density and higher breast cancer risk
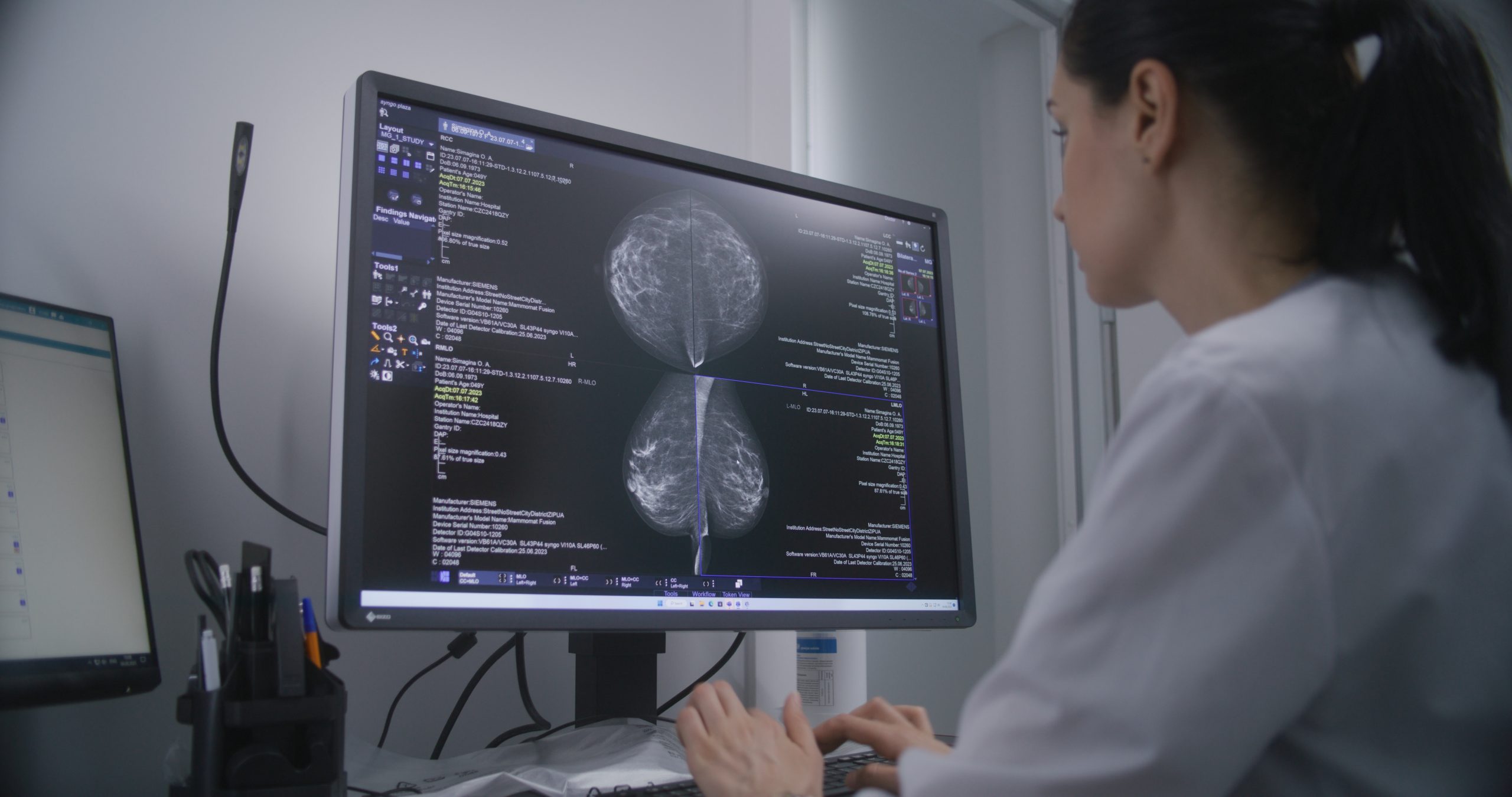
Breast density plays a significant role in both the risk of breast cancer diagnosis and the sensitivity of mammography, which may affect screening practices, new research has shown
The study showed that women with dense breasts had a 1.8 times higher likelihood of being diagnosed with breast cancer compared with women with less dense breasts.
Additionally, when accounting for screening sensitivity, the relative risk of developing breast cancer was 1.7 times higher for women with dense breasts.
The study’s lead author, Jane Lange, Ph.D. is a staff scientist at the Oregon Health & Science University Knight Cancer Institute’s Cancer Early Detection Advanced Research Center.
She said: “Breast density has been known to be associated with an increased risk of breast cancer diagnosis.
“What we wanted to do was go beyond that and determine the actual underlying risk of developing breast cancer, separate from the biases in screening.”
The study analysed data from more than 33,000 women in the Breast Cancer Surveillance Consortium from 2000 to 2018.
Researchers found that women with dense breasts were about 1.7 times more likely to develop breast cancer than those with less dense breast tissue.
Mammography is less sensitive in detecting cancer in dense breasts, leading to potential false negatives.
This issue, known as “masking,” may result in missed diagnoses and more interval cancers, where breast cancer is diagnosed between screenings.
The study found that the sensitivity of digital mammography is 73 per cent for women with extremely dense breasts, compared with 93 per cent for women with mostly fatty breasts.
Lange said: “Mammograms are less sensitive for women with dense breasts.
“That means tumors can be missed, leading to delayed detection.
“But on the other hand, women with dense breasts are often advised to get screened more frequently, which can increase the chances of finding cancer earlier.”
To account for these competing factors, the researchers used a novel statistical modeling approach to separate the actual risk of developing cancer from the likelihood of detection.
Their findings support recent updates to U.S. regulations requiring mammography centres to inform patients about their breast density and its implications for cancer risk.
The Food and Drug Administration has mandated that, starting in September 2024, all women undergoing mammograms must be notified if they have dense breasts and be advised of the potential risks.
Lange said: “Our study really reinforces the importance of these requirements.
“Even after accounting for differences in screening sensitivity, we found that breast density is strongly linked to cancer risk.”
While the study does not change current screening recommendations, Lange emphasised that women with dense breasts should be aware of their higher risk and discuss additional screening options, such as MRIs or ultrasounds, with their doctors.
The researcher said: “We’re not making specific recommendations,.
Our study shores up the evidence that breast density is directly linked to breast cancer diagnosis and onset.”
News
NHS introduces new ovarian cancer screening for high-risk women
High-risk women can now delay ovary removal surgery through NHS screening, helping them preserve fertility and avoid early surgical menopause.
The ROCA test tracks women with BRCA gene mutations every four months, monitoring cancer risk markers instead of requiring immediate preventive surgery.
University College London Hospitals NHS Foundation Trust has begun rolling out the programme, with plans for national expansion after approval from the National Institute for Health and Care Excellence (NICE).
BRCA1 and BRCA2 mutations greatly increase the risk of breast and ovarian cancer. Normally, BRCA genes repair damaged DNA, but when faulty they allow tumours to grow more easily.
Breast cancer screening with mammograms is long established, but until now there has been no equivalent for ovarian cancer. Women carrying BRCA mutations were typically advised to have their ovaries removed preventively – a step that ends fertility and triggers menopause immediately.
The ROCA test monitors changes in CA125, a blood protein that can indicate ovarian cancer when levels rise.
By testing every four months, women can keep track of their risk while delaying surgery if they wish.
Professor Adam Rosenthal, consultant gynaecologist at UCLH, said the test was designed as a damage-limitation option for women not ready for surgery.
A raised result means patients can reconsider surgery, while regular monitoring increases the chance of detecting cancer early, when outcomes are better.
Natasha Wray learned she carried the BRCA gene after breast cancer treatment at 36. She was advised to have her ovaries removed but refused.
She said: “I was 36 at the time, and I said, absolutely not. I’d just gone through seven rounds of chemo, extensive surgery, and had sort of a year and a half, two years of my life caught up in cancer treatment, and I just wanted to be left alone.
“And I also didn’t want to go through a surgical menopause in my mid-30s.
“I also very much wanted to be a mum, so I definitely wanted to hold on to any fertility that I did have.
She initially paid privately for the ROCA tests and admitted they brought anxiety.
But Rosenthal said the screening helps keep the decision about surgery at the forefront for patients.
At 41, Wray had a baby. Two years later, her test results showed raised CA125 levels, and she delayed before eventually choosing surgery.
“It wasn’t easy,” she said.
“But I do think it’s really important as well that women’s bodies aren’t just seen as baby making bodies and machines.
“I very much wanted to be a mother. That wasn’t my sole purpose for holding onto my ovaries.
“It was very personal and it actually had nothing to do with anybody else. It was very much me, how I felt in my body, what had already been felt like to me, taken from me.”
NICE has approved the ROCA test for BRCA carriers, with health officials highlighting its cost-effectiveness and aiming to make it available across England.
Cancer
HPV vaccine protects vaccinated and unvaccinated women, study finds

A large, long-term study has found that the introduction of the human papillomavirus (HPV) vaccine in community settings is highly effective in protecting young women from infections caused by the cervical-cancer-causing virus—including women who didn’t even receive the vaccine.
HPV is the most common sexually transmitted infection worldwide and is the primary cause of cervical cancer.
HPV also causes other genital cancers as well as head and neck cancers in both women and men. According to the International Agency for Research on Cancer, HPV is responsible for more than 690,000 new cancer cases each year—about 4.5 per cent of all cancers globally.
Lead author Jessica Kahn, M.D., M.P.H. is professor of paediatrics and the Dr Ernest Baden Chair in Head and Neck Pathology at Einstein.
She said: “There are two encouraging takeaways from our study.
“First, HPV vaccines work remarkably well in a real-world setting, even among women at high risk for HPV and who may not have received all vaccine doses.
“Second, we saw clear evidence of herd immunity, meaning when enough people are vaccinated, the vaccine indirectly protects unvaccinated people by reducing overall virus transmission.
“These results reinforce the potential of the HPV vaccine to prevent infection and, ultimately, eliminate cervical cancer globally.”
The research team conducted six studies in Cincinnati of 2,335 adolescent and young adult women between 2006—just before the first HPV vaccine became available—and 2023.
Participants ranged in age from 13 to 26 at enrolment.
Many reported sexual behaviours that increased risk for HPV (79 per cent had two or more male sexual partners) and 51 per cent had a history of at least one sexually transmitted infection.
Over the 17-year study period, HPV vaccination rates rose from 0 per cent to 82 per cent. As vaccination coverage increased, the rates of HPV infection dropped dramatically among vaccinated participants
Infections from HPV types covered by the 2-valent vaccine fell by 98.4 per cent
Infections from types covered by the 4-valent vaccine dropped by 94.2 per cent
Infections from types covered by the 9-valent vaccine declined by 75.7 per cent
“These outcomes show that HPV vaccines are highly effective outside of controlled trials and could dramatically reduce rates of cervical cancer and other HPV-caused cancers, including other genital cancers and head and neck cancers,” said Dr Kahn.
The researchers also found strong evidence of herd immunity.
Among unvaccinated women, infections with HPV types covered by the 2-valent vaccine decreased by 71.6 per cent.
Meanwhile, infections with HPV types covered by the 4-valent vaccine dropped by 75.8 per cent
Dr Kahn noted that the high degree of herd immunity was likely related to robust vaccination rates and vaccination of boys as well as girls.
While there wasn’t enough data yet to confirm herd protection from the more recently introduced 9-valent vaccine, the results are promising.
“In the U.S. and other countries with widespread HPV vaccination programs, cervical cancer rates are already declining,” Dr Kahn said.
“Yet in 42 countries, it remains the leading cause of cancer death among women.
“Globally, only 27 per cent of girls have received at least one dose of this lifesaving vaccine – with coverage ranging from just 1 per cent in the Eastern Mediterranean region to 68 per cent in the Americas.
“By expanding uptake of this highly safe and effective vaccine, and ensuring access to screening and treatment, we can achieve one of the greatest public health victories of our time: the elimination of cervical cancer worldwide.”
News
Ovarian cancer drug to launch in UK at US price

AbbVie said on Monday that it will launch ovarian cancer drug Elahere in the UK at the same list price as in the US.
The announcement follows President Donald Trump’s push for drugmakers to align domestic prices with the lowest levels paid by comparable high-income countries under his “most-favoured-nation” policy.
The US currently pays nearly three times more for prescription medicines than other developed nations.
AbbVie is in talks with the National Institute for Health and Care Excellence, the UK’s cost-effectiveness watchdog, to ensure the drug is valued fairly.
The company said these discussions will determine when Elahere is launched in the UK. AbbVie did not immediately respond to a request on pricing.
The move comes after Bristol Myers Squibb said last week it plans to introduce its schizophrenia drug Cobenfy in the UK next year at a price matching its US list price.
AbbVie was one of 17 drugmakers to receive letters from President Trump in July outlining how they should cut prices to match those paid overseas.
Unlike in the US, where market forces determine drug prices, European governments typically negotiate directly with companies to set prices for their health systems.
Elahere, available in the US since 2022, belongs to a new class of therapies called antibody-drug conjugates, sometimes referred to as “guided missile” drugs.
These treatments use antibodies to deliver chemotherapy directly to cancer cells with a specific protein on their surface, allowing for more precise treatment while reducing harm to healthy tissue.

 News9 hours ago
News9 hours agoMothers’, not fathers’, mental health directly linked to their children’s, study shows

 Menopause5 days ago
Menopause5 days agoNew report exposes perimenopause as biggest blind spot in women’s health

 News8 hours ago
News8 hours agoScientists turn human skin cells into eggs in IVF breakthrough

 News3 weeks ago
News3 weeks agoThe Future of femtech: Rebuilding the investment landscape

 Hormonal health1 day ago
Hormonal health1 day agoDaily pill could delay menopause ‘by years,’ study finds

 Entrepreneur4 weeks ago
Entrepreneur4 weeks agoUCL spin-out raises £2.5m to improve infant health with breast milk microbiome

 News4 weeks ago
News4 weeks agoWeightWatchers debuts menopause programme with Queen Latifah

 News4 weeks ago
News4 weeks agoWomen in UK with PCOS facing widespread failures in treatment, report finds





























